Overview
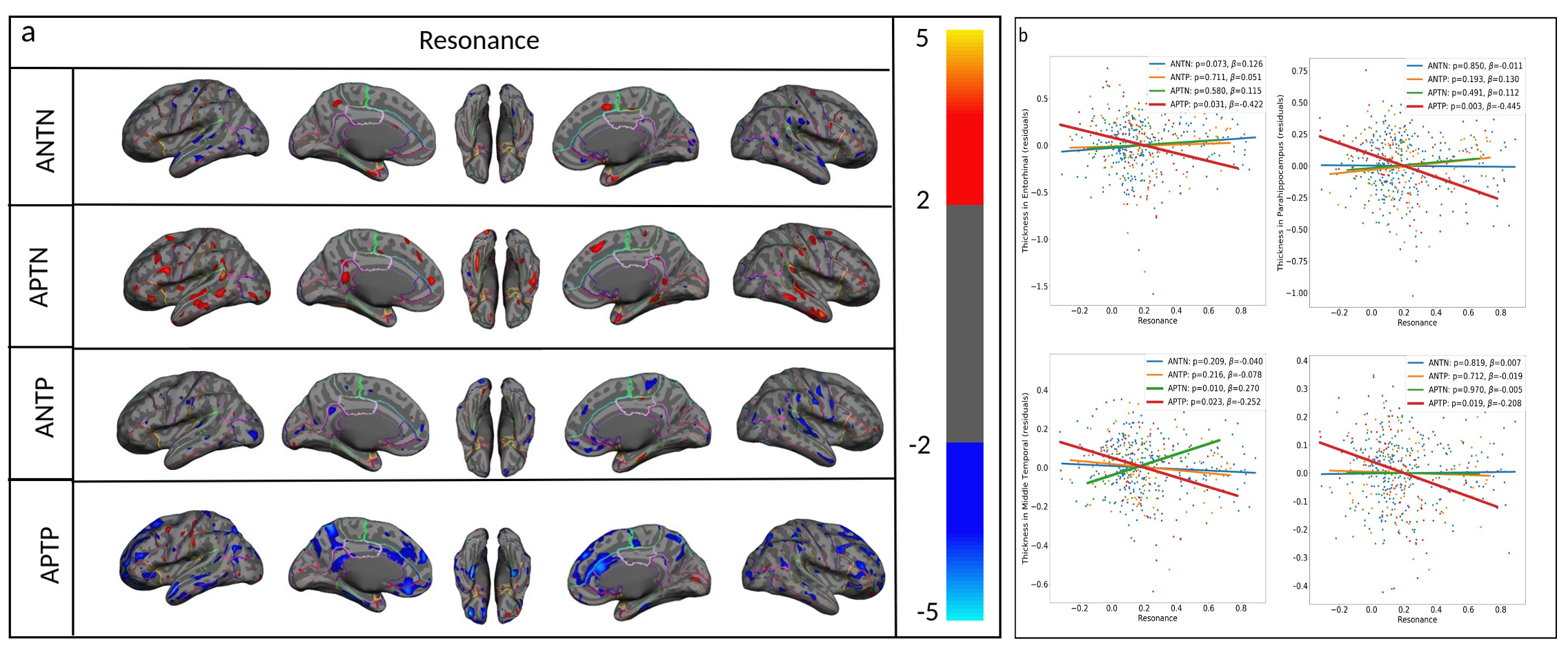
One of the most challenging issues in investigating the pathophysiology of Alzheimer’s Disease (AD) is that deposition of the AD proteins Aβ and tau can remain asymptomatic for decades or even until the end of life. Understanding why some subjects with significant brain accumulation of both Aβ and tau at autopsy showed no cognitive deficits during life has critical implications for AD treatment and prevention. We hypothesize that accumulation of tau and Aβ are independently initiated then spread gradually and mostly asymptomatically, until they spatially overlap within a brain large-scale network. Spatially-overlapping-insults initiates a resonance-point where/when a pathologic interaction accelerates their association and initiates systems-level brain network failure, neurodegeneration, and cognitive decline. Our recent report and preliminary data suggest that the maladaptive resonance between Aβ and tau starts when the two pathologies contaminate the same cortical brain regions. We will test this hypothesize by enrolling subjects younger than those enrolled in existing AD cohorts to capture the early phase of single protein deposition using 2nd generation Aβ and tau PET radiotracers. We apply advanced MRI technology, including task-based fMRI assessing both negative and positive BOLD, and detailed, domain-specific cognitive assessment, to detect and differentiate the effects of tau, Aβ and their interaction on brain structure and function with maximal sensitivity. Our key hypothesis is that Aβ but not tau disrupts resting-state activity in the default mode network, tau but not Aβ disrupts task-evoked fMRI activity, and spatially-overlapping insults (co-localized Aβ and tau) disrupts both types of activity as well as cognition. Our findings will shed light on system-level pathophysiology of AD by assessing spatiotemporal patterns of Aβ and tau progression. We will characterize the structural and functional consequences of spatially overlapping insults as a critical resonance point, after which neurodegeneration accelerates significantly. This model, if proven, suggests that even in subjects with both tau and Aβ deposition, therapeutic intervention prior to this resonance point could be an effective means to prevent neurodegeneration.
Related Publication
Hojjati SHani, Feiz F, Ozoria S, Razlighi QR. 2021. Topographical Overlapping of the Amyloid-β and Tau Pathologies in the Default Mode Network Predicts Alzheimer's Disease with Higher Specificity.. J Alzheimers Dis. 83(1):407-421.
Hojjati SHani, Butler T., Feiz F, Stern Y, Habeck C., Shteingart J., Ozoria S, Devanand D., Luchsinger J., Razlighi QR. 2022. Distinct and joint effects of low and high levels of Aβ and tau depositions on cortical thickness. submitted for review.
Funding Sources
We havs started this research study using the publicly avialable database, and our internal pilot funding from Weill Cornell Medicine. We have also applied for federal funding from NIH/NIA to be able to continue this reseach.